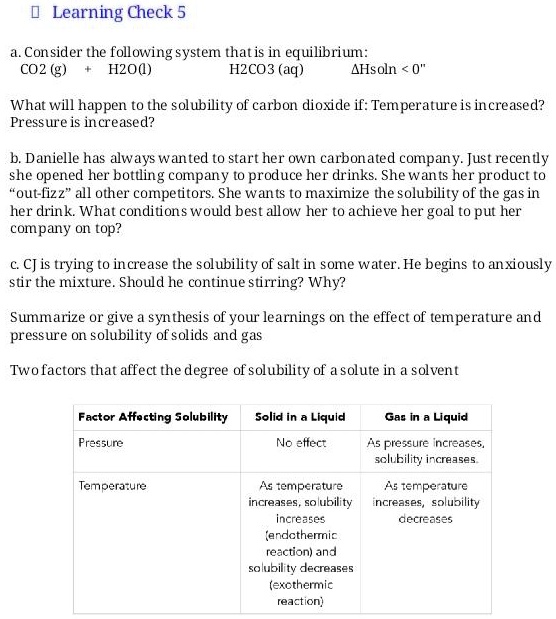
As The Pressure On The System Increases The Solubility Of Co2
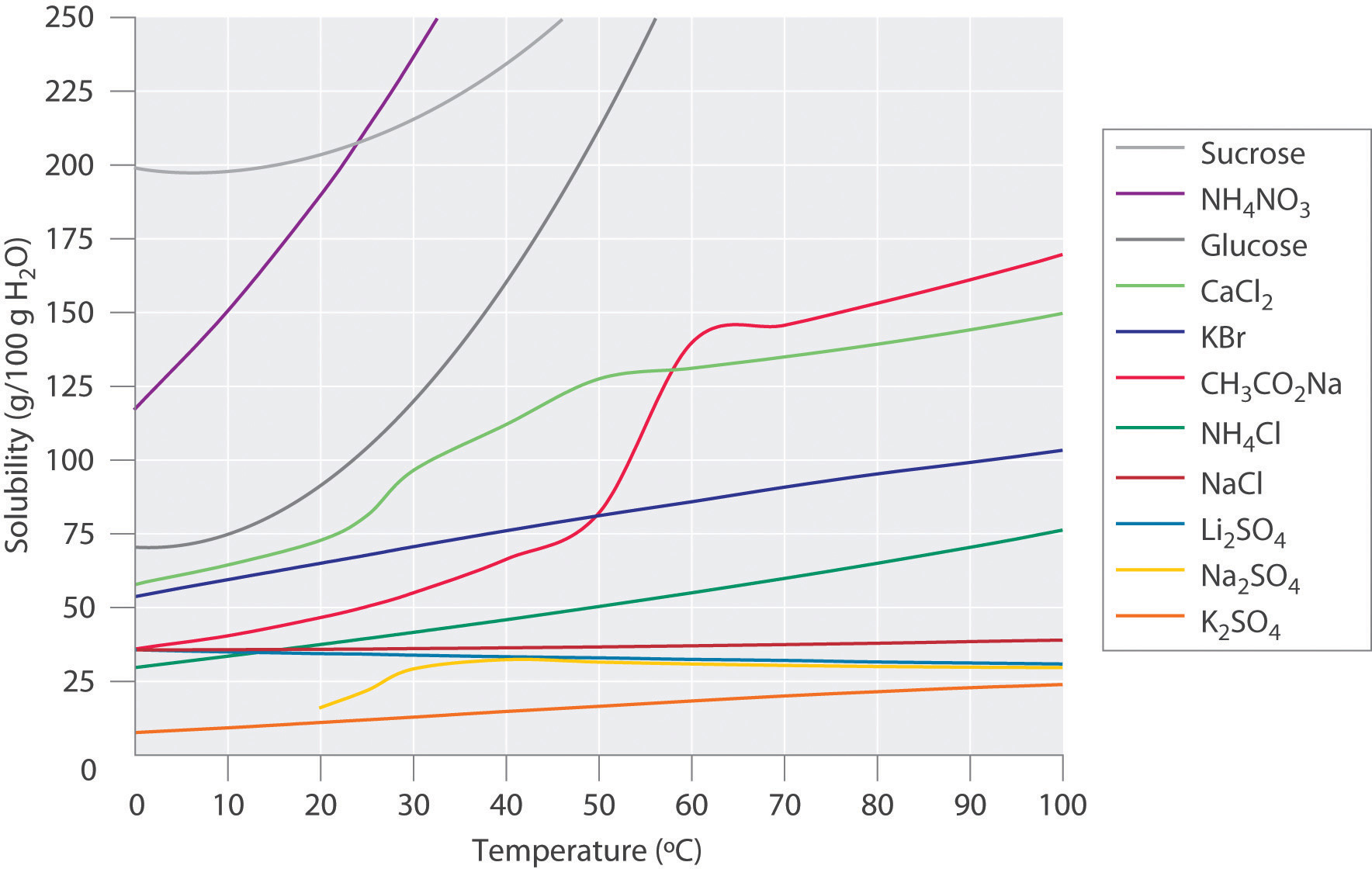
As the pressure on the system increases the solubility of co2. 1Solubility decreases with increasing temperature. This confirms theoretical calculations as well as removes previously reported ambiguities. The components of a mixture can often be separated using fractional crystallization which separates compounds according to their solubilities.
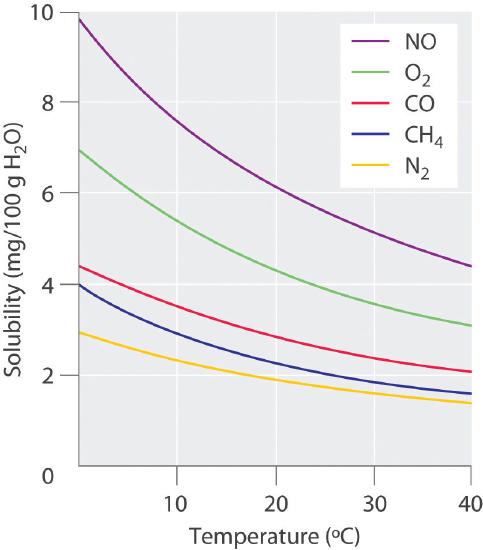
This statement is formalized in Henrys Law which states that the solubility of a gas in a liquid is directly proportional to. This is very similar to the reason that vapor pressure increases with temperature. The results of their study showed that pressure and density have straight effects on the solubility of normal alkanes in CO 2 and as the temperature and length of.
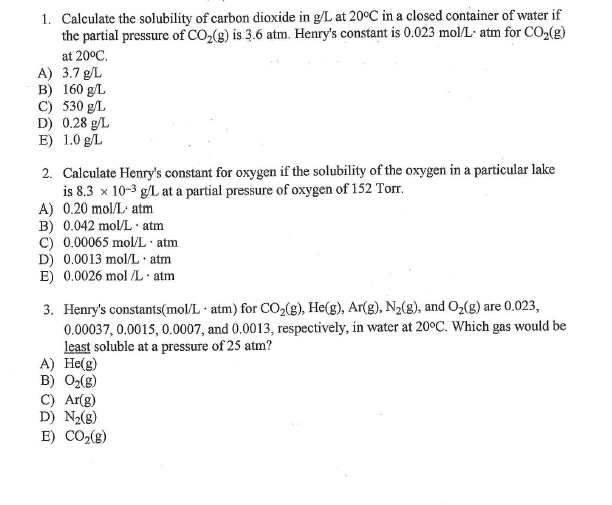
Which is the disorder of a system. 3Solubility is dependent on the surface area of the the liquid. 103 Effect of Pressure on Gas Solubility Actually the pressure dependence can be neglected as long as the pressure is not large.
At high pressures however the effect is not negligible and therefore it is necessary to consider how Henrys constant depends on pressure. Hemoglobin is capable of transporting about 23 of the CO₂ as a carbaminohemoglobin compound. The solubility of gases depends on the pressure.
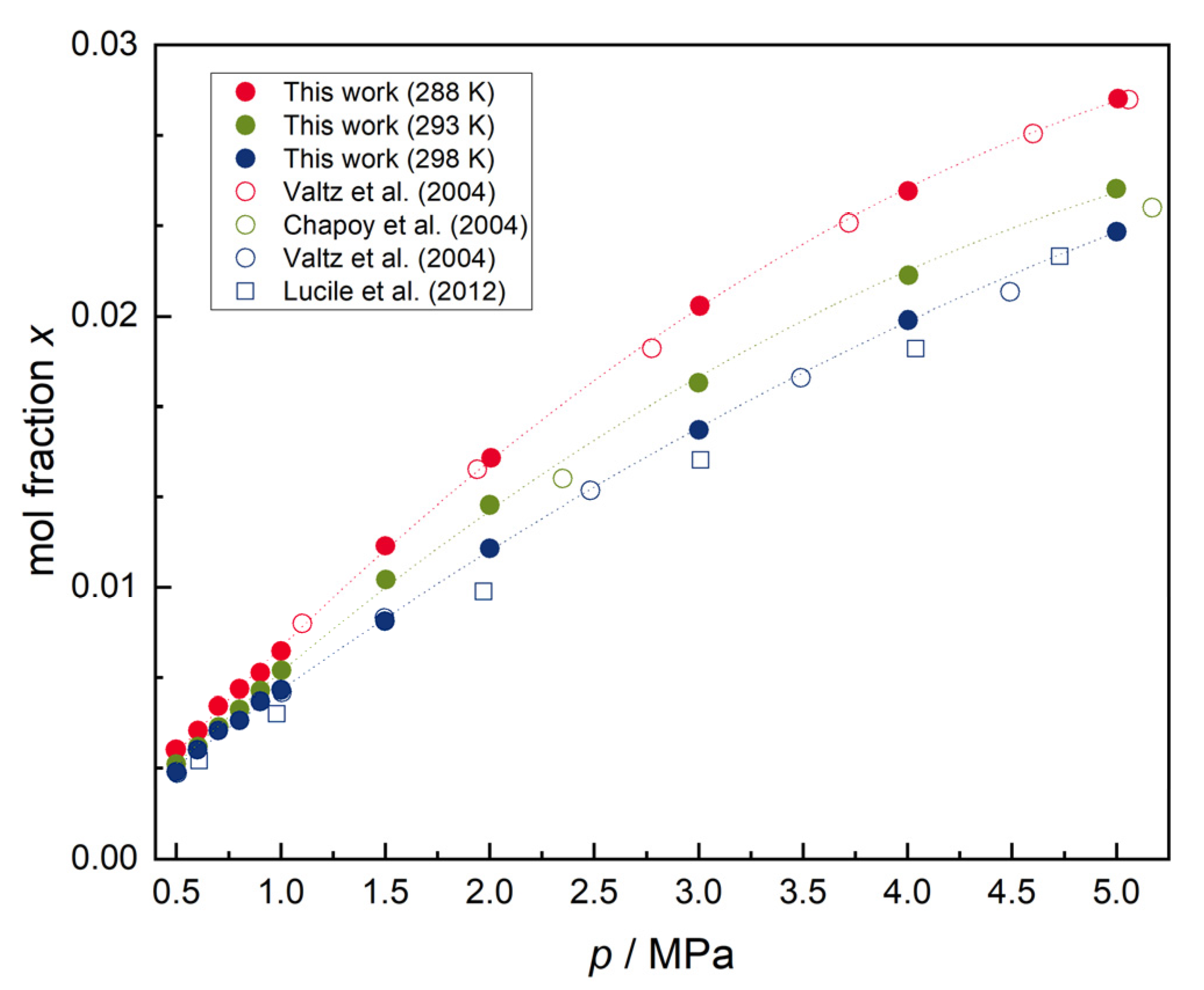
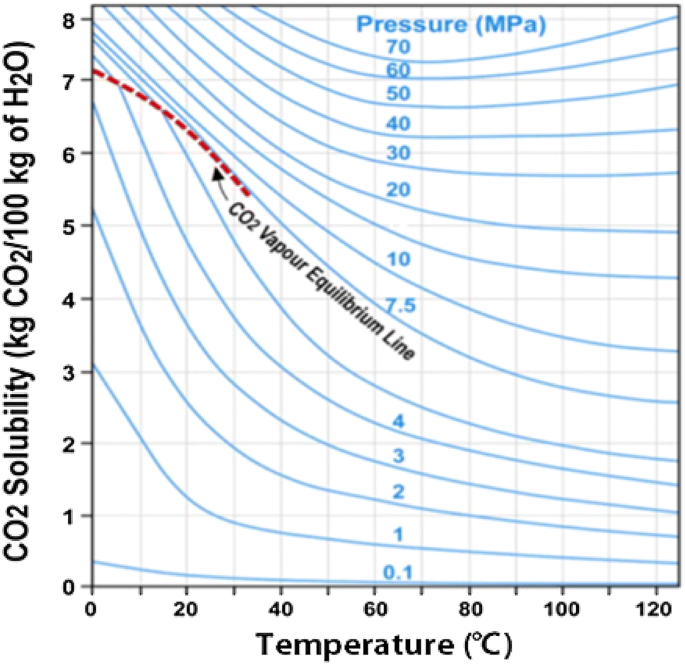
It was found that the solubility decreases with decreasing temperature in the hydrate formation region. However the solubility of CO 2 in water is much higher than that of hydrocarbon components in water. Increasing the pressure of carbon dioxide makes the reaction feasible in.
In the absence of gas hydrate the gas solubility increases with decreasing temperature but the hydrate formation process changes this trend. Adecreasing the concentration of Bdecreasing the pressure. Which change causes the equilibrium to shift to the right.
As a result the solution effervesces and some of the carbon dioxide bubbles off. All carbonated beverages are bottled under pressure to increase the carbon dioxide dissolved in solution.
The physical reason for this is that when most gases dissolve in solution the process is exothermic.
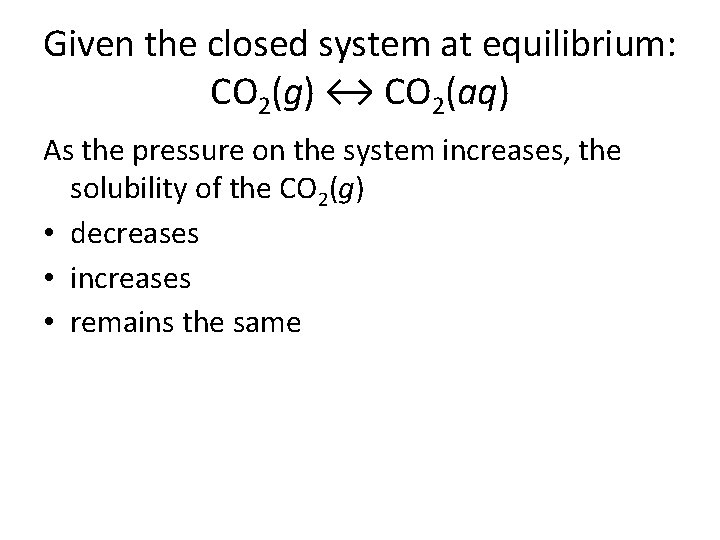
This confirms theoretical calculations as well as removes previously reported ambiguities. This is because the pressure effectively pushes the gas into the liquid. The components of a mixture can often be separated using fractional crystallization which separates compounds according to their solubilities. However the solubility of CO 2 in water is much higher than that of hydrocarbon components in water. When the bottle is opened the pressure above the solution decreases. An increase in pressure increases solubility whereas a decrease in pressure decreases solubility. The solubility of carbon dioxide in pure water in the presence of CO2 gas hydrate has been measured at temperatures between 273 and 284 K and pressures ranging from 20 to 60 bar. The solubility of CO2 in water at 0 C and 1 atm. Given the closed system at equilibrium.
From 25 literature studies and tested for their accuracy against simple thermodynamic. Note that addition of CO 2 itself does not affect Alkalinity gain 2 moles HCO 3-for every CO 3 2- Note also that capacity for CO 2 uptake determined by CO 3 2-. It was also observed. 1Solubility decreases with increasing temperature. State the general trends of the solubility of gases and solids with temperature. This confirms theoretical calculations as well as removes previously reported ambiguities. Given the closed system at equilibrium.





















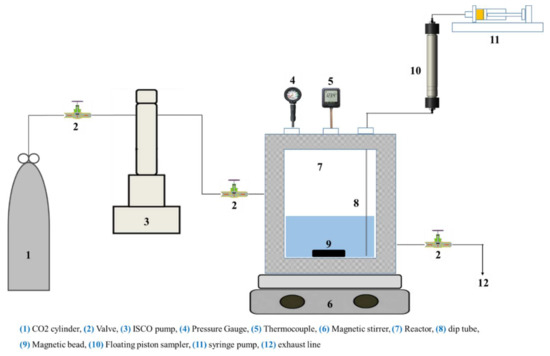
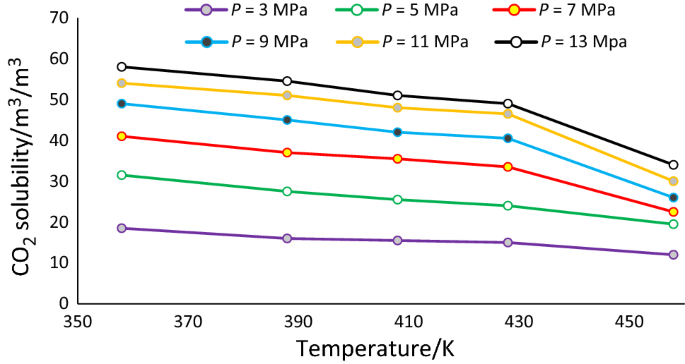


Post a Comment for "As The Pressure On The System Increases The Solubility Of Co2"